Introduction: β-thalassemia is a β-globin gene disorder resulting in decreased production of hemoglobin A and chronic anemia. Disease severity ranges from asymptomatic, mild anemia to life-long red blood cell transfusion dependence. Luspatercept is an erythroid maturation agent that reduces transfusion burden in patients with transfusion-dependent β-thalassemia. We describe real-world baseline demographics and clinical characteristics alongside treatment utilization patterns in US patients with β-thalassemia treated with luspatercept.
Methods: This retrospective, observational, cohort study used Komodo Health's Healthcare Map, a large administrative claims database in the USA. Patients with a diagnosis of β-thalassemia (ICD-10 code: D56.1) who initiated luspatercept treatment between January 1, 2020 and September 30, 2021 were included; the full study period was July 1, 2019 to September 30, 2022. The index date was defined as the first documented administration of luspatercept during the identification period. Patients were adults (≥ 18 years of age on index date) continuously enrolled for 6 months before (baseline) and 12 months after (follow-up) the index date. Discontinuation was defined as no medication claim for ≥ 90 days (assuming a 3-week schedule while taking into account potential dose delays). Descriptive statistics were used to analyze and report baseline demographics and clinical characteristics. Time-to-event variables were assessed using Kaplan-Meier methods.
Results: The 101 patients included had a mean (standard deviation [SD]) age of 37.7 (14.9) years, with a broad geographical spread throughout the USA (Midwest n = 17, 16.8%; Northeast n = 24, 23.8%; South n = 28, 27.7%; West n = 32, 31.7%). Approximately half (52.5%) were female, and mainly commercially insured (56.4%). Patients were in relatively good health (mean [SD] Charlson Comorbidity Index: 0.8 [1.7]). Bone deformities (n = 16, 15.8%), hepatosplenomegaly (n = 13, 12.9%), and thrombotic events (n = 10, 9.9%) were the most common β-thalassemia-related comorbidities observed in the baseline period. Median (interquartile range) baseline transfusion frequency during the 6-month baseline period was 4.0 (8.0), and 55.0% of the patients were transfusion dependent (≥ 4 transfusions in the 6-month baseline period).
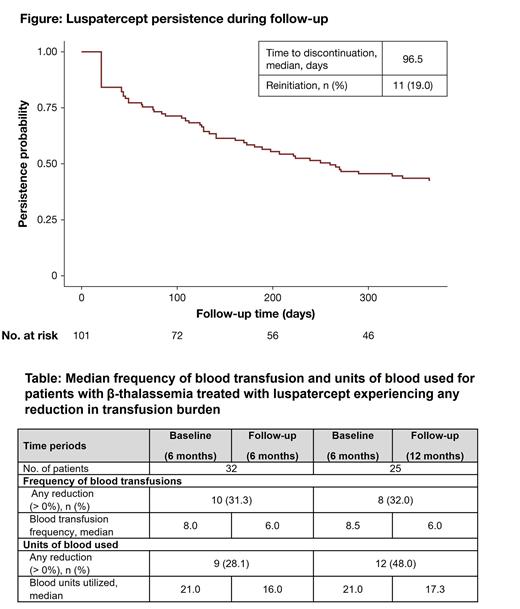
Over 40% of patients remained on luspatercept after 1 year of treatment with a median time to discontinuation of 96.5 days (Figure). Approximately one fifth (19.0%) of patients who discontinued luspatercept reinitiated treatment (Figure). Among those who were transfusion-dependent who stayed on luspatercept at 6 months, 31.3% (10/32) experienced a reduction in the frequency of transfusions, with a median decrease of 2 visits in the 6-month follow-up period from baseline, and 28.1% (9/32) observed a decrease in the units of blood transfused, with a median decrease of 5 units in the 6-month follow-up period from baseline (Table). Similarly, among patients who were transfusion-dependent who stayed on luspatercept for 12 months, 32.0% (8/25) experienced a reduction in the frequency of transfusions, with a median decrease of 2.5 visits in the follow-up period from baseline, and 48.0% (12/25) observed a decrease in the units of blood transfused, with a median decrease of 3.7 units in the 12 month follow-up period from baseline (Table).
Conclusion: This study shows that patients with β-thalassemia treated with luspatercept saw a substantial reduction in transfusion burden, in both the frequency of blood transfusion and units of blood used. As those who remained on luspatercept longer, through 12 months, saw an increased reduction in transfusion burden from baseline, these findings suggest the clinical and real-world benefit of longer-term luspatercept treatment.
Disclosures
Sheth:Bristol Myers Squibb/ Celegene: Consultancy, Other: Travel support, Research Funding; Bluebird bio: Consultancy, Other: Travel support; Fulcrum: Consultancy; Agios: Consultancy, Other: Travel support, Research Funding; CRISPR: Membership on an entity's Board of Directors or advisory committees; Chiesi: Consultancy; Vertex Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Glassberg:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Research Funding. Lee:Komodo Health: Current Employment, Current holder of stock options in a privately-held company. Ip:Komodo Health: Current Employment; Xcenda: Ended employment in the past 24 months; Statinmed: Ended employment in the past 24 months. Liu:Komodo Health: Current Employment. Moro Bueno:Bristol Myers Squibb: Current Employment, Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal